Access to dialkylated allylic stereogenic centers by Ni-catalysed enantioselective hydrovinylation of unactivated alkenes†
Abstract
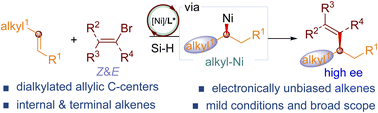
Tertiary dialkylated allylic stereogenic centers are widespread substructures in bioactive molecules and natural products. However, enantioselective access to dialkyl substituted allylic motifs remains a long-term challenge. Herein, a straightforward protocol to build allylic dialkylated stereogenic centers enabled by nickel-catalysed regio- and enantioselective hydrovinylation of isolated unactivated alkenes facilitated by a weakly coordinating group with vinyl bromides was developed, affording dialkylated allylic species in good yields with excellent enantioselectivities. The reaction distinguishes distinct alkenes and works for both terminal and internal aliphatic alkenes. The reaction proceeds under mild conditions and tolerates a wide range of functional groups.


 Please wait while we load your content...
Please wait while we load your content...