Native mass spectrometric studies of IscSU reveal a concerted, sulfur-initiated mechanism of iron–sulfur cluster assembly†
Abstract
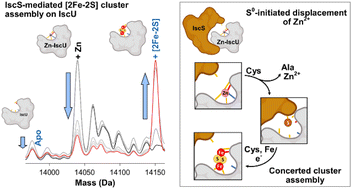
Iron–sulfur (Fe–S) clusters are cofactors essential for life. Though the proteins that function in the assembly of Fe–S clusters are well known, details of the molecular mechanism are less well established. The Isc (iron–sulfur cluster) biogenesis apparatus is widespread in bacteria and is the closest homologue to the human system. Mutations in certain components of the human system lead to disease, and so further studies of this system could be important for developing strategies for medical treatments. We have studied two core components of the Isc biogenesis system: IscS, a cysteine desulfurase; and IscU, a scaffold protein on which clusters are built before subsequent transfer onto recipient apo-proteins. Fe2+-binding, sulfur transfer, and formation of a [2Fe–2S] was followed by a range of techniques, including time-resolved mass spectrometry, and intermediate and product species were unambiguously identified through isotopic substitution experiments using 57Fe and 34S. Under cluster synthesis conditions, sulfur adducts and the [2Fe–2S] cluster product readily accumulated on IscU, but iron adducts (other than the cluster itself) were not observed at physiologically relevant Fe2+ concentrations. Our data indicate that either Fe2+ or sulfur transfer can occur first, but that the transfer of sulfane sulfur (S0) to IscU must occur first if Zn2+ is bound to IscU, suggesting that it is the key step that initiates cluster assembly. Following this, [2Fe–2S] cluster formation is a largely concerted reaction once Fe2+ is introduced.
- This article is part of the themed collection: 2023 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...