In situ modification of the d-band in the core–shell structure for efficient hydrogen storage via electrocatalytic N2 fixation†
Abstract
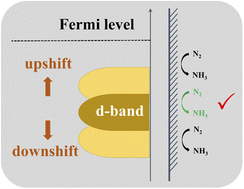
The electrochemical N2 reduction reaction (NRR) into NH3, especially powered by clean and renewable electricity, is a promising alternative to the capital- and energy-intensive Haber–Bosch process. However, the inert N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond and the frantic competition of the hydrogen evolution reaction lead to a poor NH3 yield rate and faradaic efficiency (FE). Here, we in situ construct a series of two-dimension core/shell V2O3/VN nanomeshes with a gradient nitride-layer thickness. Among them, V2O3/VN-2 exhibits the highest FE of 34.9%, an excellent NH3 yield rate of 59.7 μg h−1 mgcat.−1, and outstanding cycle stability, exceeding those of most of the NRR electrocatalysts reported to date. First-principles calculations reveal that the d-band center of VN shifts up in a nearly linear manner with the decrease of nitride-layer thickness, and V2O3/VN-2 with a d-band center closer to the Fermi level can strengthen the d–2π* coupling between the catalyst and N2 molecule, notably facilitating the N2-into-NH3 conversion.
N bond and the frantic competition of the hydrogen evolution reaction lead to a poor NH3 yield rate and faradaic efficiency (FE). Here, we in situ construct a series of two-dimension core/shell V2O3/VN nanomeshes with a gradient nitride-layer thickness. Among them, V2O3/VN-2 exhibits the highest FE of 34.9%, an excellent NH3 yield rate of 59.7 μg h−1 mgcat.−1, and outstanding cycle stability, exceeding those of most of the NRR electrocatalysts reported to date. First-principles calculations reveal that the d-band center of VN shifts up in a nearly linear manner with the decrease of nitride-layer thickness, and V2O3/VN-2 with a d-band center closer to the Fermi level can strengthen the d–2π* coupling between the catalyst and N2 molecule, notably facilitating the N2-into-NH3 conversion.


 Please wait while we load your content...
Please wait while we load your content...