Understanding the electrocatalytic mechanism of self-template formation of hierarchical Co9S8/Ni3S2 heterojunctions for highly selective electroreduction of nitrobenzene†
Abstract
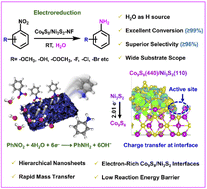
Aqueous electrochemical nitroarene reduction reaction using H2O as the sustainable hydrogen source is an emerging technology to produce functionalized anilines. However, the development of low-cost electrocatalysts and the fundamental mechanistic understanding of the selective NO-RR still remain challenging. Herein, self-supporting hierarchical nanosheets consisting of high-density Co9S8/Ni3S2 heterojunctions on Ni foam (Co9S8/Ni3S2-NF) are constructed via an in situ self-template strategy. With combined advantages of high-loading, high surface exposure, efficient conductivity and unique electronic structure of the Co9S8/Ni3S2 interface, the as-prepared Co9S8/Ni3S2-NF exhibits efficient electrocatalytic NO-RR performance, including up to 99.0% conversion and 96.0% selectivity towards aniline, and outstanding functional group tolerance. Mechanistic investigations and theoretical calculations reveal that electron transfer from Ni3S2 to Co9S8 is beneficial for the co-adsorption of H2O and nitrobenzene molecules at the interfacial sites, promoting the formation of active hydrogen and subsequent reduction of nitrobenzene. Additionally, the interfacial charge transfer breaks the symmetry of two active Co sites at the Co9S8/Ni3S2 interface, which markedly reduces the energy barrier for reduction of nitrobenzene to aniline. This work offers a successful example for the interfacial engineering of metal sulfide-based heterojunctions with excellent electrocatalytic nitroarene reduction performance, and also paves the way for the in-depth understanding of the corresponding mechanism.


 Please wait while we load your content...
Please wait while we load your content...