An iron(ii)-based metalloradical system for intramolecular amination of C(sp2)–H and C(sp3)–H bonds: synthetic applications and mechanistic studies†
Abstract
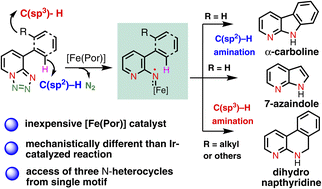
A catalytic system for intramolecular C(sp2)–H and C(sp3)–H amination of substituted tetrazolopyridines has been successfully developed. The amination reactions are developed using an iron-porphyrin based catalytic system. It has been demonstrated that the same iron-porphyrin based catalytic system efficiently activates both the C(sp2)–H and C(sp3)–H bonds of the tetrazole as well as azide-featuring substrates with a high level of regioselectivity. The method exhibited an excellent functional group tolerance. The method affords three different classes of high-value N-heterocyclic scaffolds. A number of important late-stage C–H aminations have been performed to access important classes of molecules. Detailed studies (experimental and computational) showed that both the C(sp2)–H and C(sp3)–H amination reactions involve a metalloradical activation mechanism, which is different from the previously reported electro-cyclization mechanism. Collectively, this study reports the discovery of a new class of metalloradical activation modes using a base metal catalyst that should find wide application in the context of medicinal chemistry, drug discovery and industrial applications.


 Please wait while we load your content...
Please wait while we load your content...