N-Terminal cysteine mediated backbone-side chain cyclization for chemically enhanced phage display†
Abstract
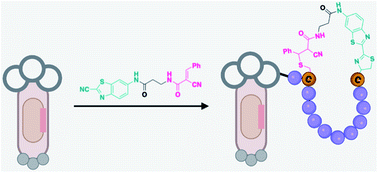
Phage display, an ingenious invention for evaluating peptide libraries, has been limited to natural peptides that are ribosomally assembled with proteinogenic amino acids. Recently, there has been growing interest in chemically modifying phage libraries to create nonnatural cyclic and multicyclic peptides, which are appealing for use as inhibitors of protein–protein interactions. While earlier reports largely focused on side-chain side-chain cyclization, we report herein a novel strategy for creating backbone-side chain cyclized peptide libraries on phage. Our strategy capitalizes on the unique reactivity of an N-terminal cysteine (NCys) with 2-cyanobenzothiazole (CBT) which, in conjugation with another thiol-reactive group, can elicit rapid cyclization between an NCys and an internal cysteine. The resulting library was screened against two model proteins, namely Keap1 and Sortase A. The screening readily revealed potent inhibitors for both proteins with certain Keap1 ligands reaching low nanomolar potency. The backbone-side chain cyclization strategy described herein presents a significant addition to the toolkit of creating nonnatural macrocyclic peptide libraries for phage display.


 Please wait while we load your content...
Please wait while we load your content...