Visible-light-induced cross-coupling of aryl iodides with hydrazones via an EDA-complex†
Abstract
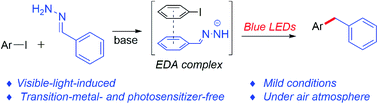
A visible-light-induced, transition-metal and photosensitizer-free cross-coupling of aryl iodides with hydrazones was developed. In this strategy, hydrazones were used as alternatives to organometallic reagents, in the absence of a transition metal or an external photosensitizer, making this cross-coupling mild and green. The protocol was compatible with a variety of functionalities, including methyl, methoxy, trifluoromethyl, halogen, and heteroaromatic rings. Mechanistic investigations showed that the association of the hydrazone anion with aryl halides formed an electron donor–acceptor complex, which when excited with visible light generated an aryl radical via single-electron transfer.


 Please wait while we load your content...
Please wait while we load your content...