Enantioselective assembly and recognition of heterochiral porous organic cages deduced from binary chiral components†
Abstract
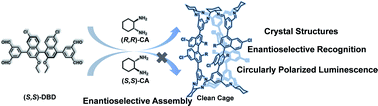
Chiral recognition and discrimination is not only of significance in biological processes but also a powerful method to fabricate functional supramolecular materials. Herein, a pair of heterochiral porous organic cages (HPOC-1), out of four possible enantiomeric products, with mirror stereoisomeric crystal structures were cleanly prepared by condensation occurring in the exclusive combination of cyclohexanediamine and binaphthol-based tetraaldehyde enantiomers. Nuclear magnetic resonance and luminescence spectroscopy have been employed to monitor the assembly process of HPOC-1, revealing the clean formation of heterochiral organic cages due to the enantioselective recognition of (S,S)-binaphthol towards (R,R)-cyclohexanediamine derivatives and vice versa. Interestingly, HPOC-1 exhibits circularly polarized luminescence and enantioselective recognition of chiral substrates according to the circular dichroism spectral change. Theoretical simulations have been carried out, rationalizing both the enantioselective assembly and recognition of HPOC-1.


 Please wait while we load your content...
Please wait while we load your content...