Enantioselective synthesis of (−)-tetrabenazine via continuous crystallization-induced diastereomer transformation†
Abstract
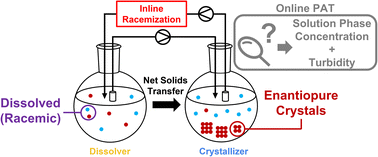
A multi-well continuous CIDT approach with inline racemization of the solution phase is presented. Using two in-house built PATs and a flow reactor, we were able to successfully crystallize an enantiopure salt of TBZ, the active metabolite of the tardive dyskinesia drug valbenazine. Despite discovering an undesired racemic solid phase, inline racemization combined with careful control of crystallization conditions allowed for multigram quantities of enantiopure material to be harvested using our setup. Critically, this control was made possible by the use of PATs to observe and quantify the composition of both the solid and solution phases.


 Please wait while we load your content...
Please wait while we load your content...