Bare and ligand protected planar hexacoordinate silicon in SiSb3M3+ (M = Ca, Sr, Ba) clusters†
Abstract
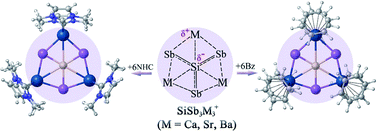
The occurrence of planar hexacoordination is very rare in main group elements. We report here a class of clusters containing a planar hexacoordinate silicon (phSi) atom with the formula SiSb3M3+ (M = Ca, Sr, Ba), which have D3h (1A1′) symmetry in their global minimum structure. The unique ability of heavier alkaline-earth atoms to use their vacant d atomic orbitals in bonding effectively stabilizes the peripheral ring and is responsible for covalent interaction with the Si center. Although the interaction between Si and Sb is significantly stronger than the Si–M one, sizable stabilization energies (−27.4 to −35.4 kcal mol−1) also originated from the combined electrostatic and covalent attraction between Si and M centers. The lighter homologues, SiE3M3+ (E = N, P, As; M = Ca, Sr, Ba) clusters, also possess similar D3h symmetric structures as the global minima. However, the repulsive electrostatic interaction between Si and M dominates over covalent attraction making the Si–M contacts repulsive in nature. Most interestingly, the planarity of the phSi core and the attractive nature of all the six contacts of phSi are maintained in N-heterocyclic carbene (NHC) and benzene (Bz) bound SiSb3M3(NHC)6+ and SiSb3M3(Bz)6+ (M = Ca, Sr, Ba) complexes. Therefore, bare and ligand-protected SiSb3M3+ clusters are suitable candidates for gas-phase detection and large-scale synthesis, respectively.
- This article is part of the themed collections: Most popular 2022 physical and theoretical chemistry articles and 2022 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...