Accelerated interfacial proton transfer for promoting electrocatalytic activity†
Abstract
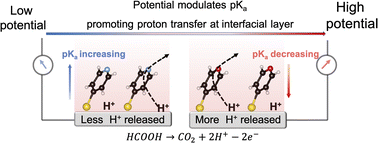
Interfacial pH is critical to electrocatalytic reactions involving proton-coupled electron transfer (PCET) processes, and maintaining an optimal interfacial pH at the electrochemical interface is required to achieve high activity. However, the interfacial pH varies inevitably during the electrochemical reaction owing to slow proton transfer at the interfacial layer, even in buffer solutions. It is therefore necessary to find an effective and general way to promote proton transfer for regulating the interfacial pH. In this study, we propose that promoting proton transfer at the interfacial layer can be used to regulate the interfacial pH in order to enhance electrocatalytic activity. By adsorbing a bifunctional 4-mercaptopyridine (4MPy) molecule onto the catalyst surface via its thiol group, the pyridyl group can be tethered on the electrochemical interface. The pyridyl group acts as both a good proton acceptor and donor for promoting proton transfer at the interfacial layer. Furthermore, the pKa of 4MPy can be modulated with the applied potentials to accommodate the large variation of interfacial pH under different current densities. By in situ electrochemical surface-enhanced Raman spectroscopy (in situ EC-SERS), we quantitatively demonstrate that proton transfer at the interfacial layer of the Pt catalyst coated with 4MPy (Pt@4MPy) remains ideally thermoneutral during the H+ releasing electrocatalytic oxidation reaction of formic acid (FAOR) at high current densities. Thus, the interfacial pH is controlled effectively. In this way, the FAOR apparent current measured from Pt@4MPy is twice that measured from a pristine Pt catalyst. This work establishes a general strategy for regulating interfacial pH to enhance the electrocatalytic activities.


 Please wait while we load your content...
Please wait while we load your content...