Revealing hydrogen spillover pathways in reducible metal oxides†
Abstract
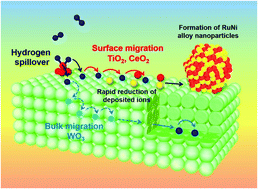
Hydrogen spillover, the migration of dissociated hydrogen atoms from noble metals to their support materials, is a ubiquitous phenomenon and is widely utilized in heterogeneous catalysis and hydrogen storage materials. However, in-depth understanding of the migration of spilled hydrogen over different types of supports is still lacking. Herein, hydrogen spillover in typical reducible metal oxides, such as TiO2, CeO2, and WO3, was elucidated by combining systematic characterization methods involving various in situ techniques, kinetic analysis, and density functional theory calculations. TiO2 and CeO2 were proven to be promising platforms for the synthesis of non-equilibrium RuNi binary solid solution alloy nanoparticles displaying a synergistic promotional effect in the hydrolysis of ammonia borane. Such behaviour was driven by the simultaneous reduction of both metal cations under a H2 atmosphere over TiO2 and CeO2, in which hydrogen spillover favorably occurred over their surfaces rather than within their bulk phases. Conversely, hydrogen atoms were found to preferentially migrate within the bulk prior to the surface over WO3. Thus, the reductions of both metal cations occurred individually on WO3, which resulted in the formation of segregated NPs with no activity enhancement.


 Please wait while we load your content...
Please wait while we load your content...