An unprecedented azobenzene-based organic single-component ferroelectric†
Abstract
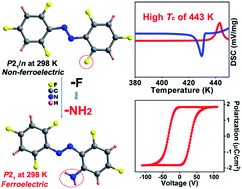
Organic single-component ferroelectrics, as an important class of metal-free ferroelectrics, are highly desirable because of their easy processing, mechanical flexibility, and biocompatibility. However, although nearly 50 years have passed since the discovery of photochromism in azobenzene-doped cholesteric liquid crystals, ferroelectricity has never been found in azobenzene-based crystals. Here, we use an amino group to substitute a fluorine atom of 2,2′,4,4′,6,6′-hexafluoroazobenzene, which successfully introduces ferroelectricity into 2-amino-2′,4,4′,6,6′-pentafluoroazobenzene (APFA). APFA shows an extremely high Curie temperature (Tc) of 443 K, which is outstanding among single-component ferroelectrics. It also exhibits an indirect optical band gap of 2.27 eV as well as photoisomerization behavior between the trans-form and the cis-form triggered by pedal motion. To our knowledge, APFA is the first azobenzene-based ferroelectric crystal. This work opens an avenue to design excellent single-component ferroelectrics and will inspire the exploration of azobenzene-based ferroelectrics for promising applications in biofriendly ferroelectric devices.
- This article is part of the themed collection: Most popular 2022 materials and energy articles


 Please wait while we load your content...
Please wait while we load your content...