Controllable access to trifluoromethyl-containing indoles and indolines: palladium-catalyzed regioselective functionalization of unactivated alkenes with trifluoroacetimidoyl chlorides†
Abstract
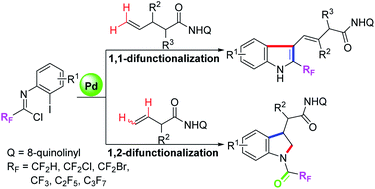
The synthesis of diverse products from the same starting materials is always attractive in organic chemistry. Here, a palladium-catalyzed substrate-controlled regioselective functionalization of unactivated alkenes with trifluoroacetimidoyl chlorides has been developed, which provides a direct but controllable access to a variety of structurally diverse trifluoromethyl-containing indoles and indolines. In more detail, with respect to γ,δ-alkenes, 1,1-geminal difunctionalization of unactivated alkenes with trifluoroacetimidoyl chloride enables the [4 + 1] annulation to produce indoles; as for β,γ-alkenes, a [3 + 2] heteroannulation with the hydrolysis product of trifluoroacetimidoyl chloride through 1,2-vicinal difunctionalization of alkenes occurs to deliver indoline products. The structure of alkene substrates differentiates the regioselectivity of the reaction.


 Please wait while we load your content...
Please wait while we load your content...