Asymmetrically bridged aroyl-S,N-ketene acetal-based multichromophores with aggregation-induced tunable emission†
Abstract
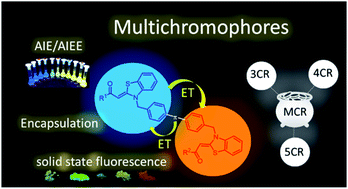
Asymmetrically bridged aroyl-S,N-ketene acetals and aroyl-S,N-ketene acetal multichromophores can be readily synthesized in consecutive three-, four-, or five-component syntheses in good to excellent yields by several successive Suzuki-couplings of aroyl-S,N-ketene acetals and bis(boronic)acid esters. Different aroyl-S,N-ketene acetals as well as linker molecules yield a library of 23 multichromophores with substitution and linker pattern-tunable emission properties. This allows control of different communication pathways between the chromophores and of aggregation-induced emission (AIE) and energy transfer (ET) properties, providing elaborate aggregation-based fluorescence switches.


 Please wait while we load your content...
Please wait while we load your content...