Torsional strain inversed chemoselectivity in a Pd-catalyzed atroposelective carbonylation reaction of dibenzothiophenium†
Abstract
A palladium-catalyzed enantioselective ring-opening/carbonylation of cyclic diarylsulfonium salts is reported. In comparison to thioethers, the sulfonium salts displayed high reactivity and enabled the reaction to be performed under mild conditions (room temperature). The steric repulsion of the two non-hydrogen substituents adjacent to the axis led cyclic diarylsulfonium salts to be distorted, which enabled the ring-opening reaction to proceed with significant preference for breaking the exocyclic C–S bond.
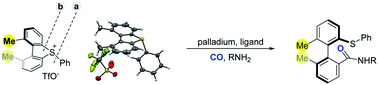


 Please wait while we load your content...
Please wait while we load your content...