Isolation of the elusive bisbenzimidazole Bbim3−˙ radical anion and its employment in a metal complex†
Abstract
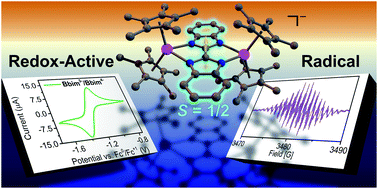
The discovery of singular organic radical ligands is a formidable challenge due to high reactivity arising from the unpaired electron. Matching radical ligands with metal ions to engender magnetic coupling is crucial for eliciting preeminent physical properties such as conductivity and magnetism that are crucial for future technologies. The metal-radical approach is especially important for the lanthanide ions exhibiting deeply buried 4f-orbitals. The radicals must possess a high spin density on the donor atoms to promote strong coupling. Combining diamagnetic 89Y (I = 1/2) with organic radicals allows for invaluable insight into the electronic structure and spin-density distribution. This approach is hitherto underutilized, possibly owing to the challenging synthesis and purification of such molecules. Herein, evidence of an unprecedented bisbenzimidazole radical anion (Bbim3−˙) along with its metalation in the form of an yttrium complex, [K(crypt-222)][(Cp*2Y)2(μ-Bbim˙)] is provided. Access of Bbim3−˙ was feasible through double-coordination to the Lewis acidic metal ion and subsequent one-electron reduction, which is remarkable as Bbim2− was explicitly stated to be redox-inactive in closed-shell complexes. Two molecules containing Bbim2− (1) and Bbim3−˙ (2), respectively, were thoroughly investigated by X-ray crystallography, NMR and UV/Vis spectroscopy. Electrochemical studies unfolded a quasi-reversible feature and emphasize the role of the metal centre for the Bbim redox-activity as neither the free ligand nor the Bbim2− complex led to analogous CV results. Excitingly, a strong delocalization of the electron density through the Bbim3−˙ ligand was revealed via temperature-dependent EPR spectroscopy and confirmed through DFT calculations and magnetometry, rendering Bbim3−˙ an ideal candidate for single-molecule magnet design.
- This article is part of the themed collection: 2022 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...