Diboramacrocycles: reversible borole dimerisation–dissociation systems†
Abstract
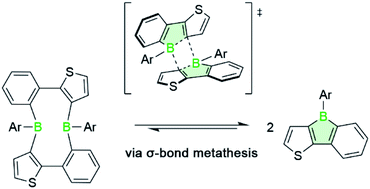
We report that the outcome of the tin–boron exchange reaction of a mixed thiophene-benzo-fused stannole with aryldibromoboranes is associated with the steric bulk of the aryl substituent of the borane reagent, leading to either boroles or large diboracycles as products. NMR spectroscopic studies indicate that the two products can reversibly interconvert in solution, and mechanistic density functional theory (DFT) calculations reveal boroles to be intermediates in the formation of the diboracyclic products. The addition of Lewis bases to the diboracycles leads to the corresponding borole adducts, demonstrating that they react as “masked” boroles. Additionally, the reaction of the title compounds with a series of organic azides affords complex heteropropellanes, formally 2 : 1 borole-azide adducts, that deviate from the usual BN aromatic compounds formed via nitrogen atom insertion into the boroles.
- This article is part of the themed collection: 2022 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...