Light-driven reduction of aromatic olefins in aqueous media catalysed by aminopyridine cobalt complexes†
Abstract
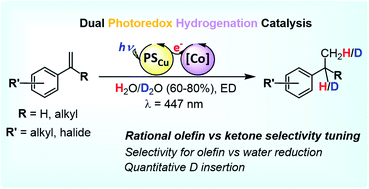
A catalytic system based on earth-abundant elements that efficiently hydrogenates aryl olefins using visible light as the driving-force and H2O as the sole hydrogen atom source is reported. The catalytic system involves a robust and well-defined aminopyridine cobalt complex and a heteroleptic Cu photoredox catalyst. The system shows the reduction of styrene in aqueous media with a remarkable selectivity (>20 000) versus water reduction (WR). Reactivity and mechanistic studies support the formation of a [Co–H] intermediate, which reacts with the olefin via a hydrogen atom transfer (HAT). Synthetically useful deuterium-labelled compounds can be straightforwardly obtained by replacing H2O with D2O. Moreover, the dual photocatalytic system and the photocatalytic conditions can be rationally designed to tune the selectivity for aryl olefin vs. aryl ketone reduction; not only by changing the structural and electronic properties of the cobalt catalysts, but also by modifying the reduction properties of the photoredox catalyst.
- This article is part of the themed collections: Most popular 2022 catalysis articles and 2022 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...