Asymmetric addition of Grignard reagents to ketones: culmination of the ligand-mediated methodology allows modular construction of chiral tertiary alcohols†
Abstract
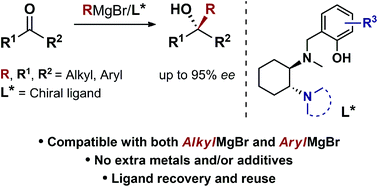
A new class of biaryl chiral ligands derived from 1,2-diaminocyclohexane (1,2-DACH) has been designed to enable the asymmetric addition of aliphatic and, for the first time, aromatic Grignard reagents to ketones for the preparation of highly enantioenriched tertiary alcohols (up to 95% ee). The newly developed ligands L12 and L12′ together with the previously reported L0 and L0′ define a set of complementary chiral promoters, which provides access to the modular construction of a broad range of structurally diverse non-racemic tertiary alcohols, bearing challenging quaternary stereocenters. The present advancements bring to completion our asymmetric Grignard methodology by expanding the scope to aromatic organomagnesium reagents, while facilitating its implementation in organic synthesis thanks to improved synthetic routes for the straightforward access to the chiral ligands. The synthetic utility of the method has been demonstrated by the development of a novel and highly enantioselective formal synthesis of the antihistamine API clemastine via intermediate (R)-3a. Exploiting the power of the 3-disconnection approach offered by the Grignard synthesis, (R)-3a is obtained in 94% ee with ligand (R,R)-L12. The work described herein marks the finalization of our ongoing effort towards the establishment of an effective and broadly applicable methodology for the asymmetric Grignard synthesis of chiral tertiary alcohols.

 Please wait while we load your content...
Please wait while we load your content...