An organophotocatalytic late-stage N–CH3 oxidation of trialkylamines to N-formamides with O2 in continuous flow†
Abstract
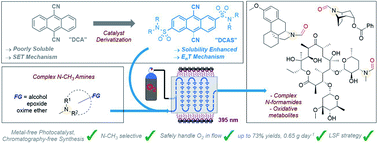
We report an organophotocatalytic, N–CH3-selective oxidation of trialkylamines in continuous flow. Based on the 9,10-dicyanoanthracene (DCA) core, a new catalyst (DCAS) was designed with solubilizing groups for flow processing. This allowed O2 to be harnessed as a sustainable oxidant for late-stage photocatalytic N–CH3 oxidations of complex natural products and active pharmaceutical ingredients bearing functional groups not tolerated by previous methods. The organophotocatalytic gas–liquid flow process affords cleaner reactions than in batch mode, in short residence times of 13.5 min and productivities of up to 0.65 g per day. Spectroscopic and computational mechanistic studies showed that catalyst derivatization not only enhanced solubility of the new catalyst compared to poorly-soluble DCA, but profoundly diverted the photocatalytic mechanism from singlet electron transfer (SET) reductive quenching with amines toward energy transfer (EnT) with O2.
- This article is part of the themed collection: Most popular 2021 catalysis articles, 2021


 Please wait while we load your content...
Please wait while we load your content...