Production of pentyl valerate from γ-valerolactone, pentanol and H2 using Pd and Rh-based bifunctional catalysts
Abstract
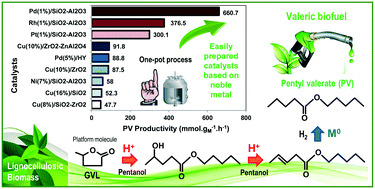
The production of pentyl valerate (PV) from γ-valerolactone (GVL) in the liquid phase in the presence of pentanol and H2 over Rh and Pd-based catalysts, using SiO2–Al2O3 (SA) as a support, was studied in this work. Both catalysts had metal loadings lower than 1 wt% and were prepared by incipient wetness impregnation using chlorinated metal precursors. The M0/SA catalysts exhibited a specific surface area and pore volume slightly lower than those of the SA support and the presence of metal nanoparticles with an average size of 5.6 nm for Rh/SA and 3.7 nm for Pd/SA. A total acid site density 10% lower than that of SA was determined for Rh/SA, whereas a value 45% higher was observed for Pd/SA. The relative strength of acid sites followed the pattern: Pd/SA > Rh/SA > SA with mainly Lewis acid sites in all the cases. Pd/SA showed a higher activity, selectivity to PV and carbon balance than Rh/SA. These results indicate that both a relatively high acid site density and smaller metal particles promote the conversion of adsorbed intermediates, reaching a GVL conversion of 81.1% and a PV yield of 70.1% over Pd/SA after 8 h. Therefore, PV productivity values 25% and 120% higher than the best reported value were reached over Rh/SA and Pd/SA, respectively, showing the potential of these catalysts to convert GVL into biofuels for transportation purposes. Finally, with Pd/SA, the apparent activation energy was equal to 25.6 kcal mol−1, the reaction order for H2 was about zero and a unitary reaction order for GVL was determined.


 Please wait while we load your content...
Please wait while we load your content...