A one-pot biocatalytic and organocatalytic cascade delivers high titers of 2-ethyl-2-hexenal from n-butanol†
Abstract
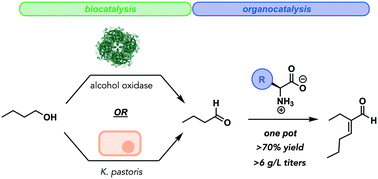
Biocatalysis provides facile access to selective chemical transformations and helps satisfy sustainable chemical production criteria. However, the reaction scope of biocatalysts is significantly narrower compared to synthetic chemical transformations. Hybrid biocatalytic–chemocatalytic cascades expand the scope of products while maintaining many of the benefits associated with biocatalysis. Here, we report that single-pot systems with whole cell K. pastoris (ATCC® 28485™) or isolated enzyme alcohol oxidase (E.C. 1.1.3.13) as oxidative biocatalysts with a lysine organocatalyst yield the commercial target, 2-ethyl-2-hexenal (2-EH) from n-butanol in a two-step hybrid cascade. Peak yields for both biocatalysts were achieved with 100 mM n-butanol at pH 8 and 30 °C. The isolated enzyme slightly outperformed whole cell K. pastoris, reaching 73% conversion (4.7 g L−1 titers) compared to 61% (3.9 g L−1 titers) in whole cell systems. Titers could be improved for both biocatalysts (5.7–6.7 g L−1) at increased butanol loading; however, this came at the expense of decreased yields. Compared to our initial results with a Gluconobacter oxidans whole cell biocatalyst, the reported system improves upon 2-EH titers by 2.8–3.3-fold at maximal yields.


 Please wait while we load your content...
Please wait while we load your content...