Mechanistic investigations on a homogeneous ruthenium Guerbet catalyst in a flow reactor†
Abstract
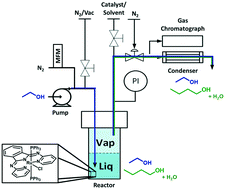
A mechanistic investigation on the ethanol self-condensation reaction (Guerbet reaction) catalyzed by a bis(pyridylimino)isoindolate Ru(II) catalyst was performed using a specifically designed continuously-stirred tank reactor (CSTR). Leveraging vapor–liquid equilibrium, the homogeneous catalyst was maintained in the reactor at a constant concentration by dissolving it in a non-volatile solvent while volatile substrates were fed continuously. The activity of the catalyst was monitored by analyzing the vapor exiting the reactor (reagents and products) using an in-line gas chromatograph. The formation of C6 products demonstrates the catalyst's reactivity towards butanol, and the detection of solely saturated products implies that hydrogenation is fast under the reaction conditions. These observations led us to perform a detailed study of the hydrogenation step that provided evidence for a hydrogen-transfer pathway. The corresponding reaction mechanism for the Guerbet reaction was established.


 Please wait while we load your content...
Please wait while we load your content...