How the substrate affects amination reaction kinetics of nitrochlorobenzene†
Abstract
The reaction rate of the amination is affected by its electron-withdrawing group, however, there is currently a lack of quantitative research on the reaction rate of different substrates in experiments and simulations. In this work, taking the amination of nitrochlorobenzene as an example, the effects of various substrates, namely 4-nitrchlorobenzene, 4-chloro-3-(trifluoromethyl)nitrobenzene, 2,4-dinitrochloroben-zene, and 3,4-dichloronitrobenzene, on the reaction rate were systematically examined in a microflow system. Through the homogeneous continuous amination reaction strategy, the reaction kinetic characteristics of different substrates were obtained. The stronger the electron-withdrawing ability of the side chain group, the milder the reaction conditions and the shorter the reaction time. Furthermore, a density functional theory (DFT) simulation method was established to calculate the condensed local electrophilicity index (ωA). There is a good fitting on the relationship between the relative reaction rate 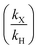 of different substrates and the relative condensed local electrophilic index (ωAX − ωAH). It not only allows the prediction of the reaction rate of different substrates and the design of industrial production systems but also inspires us to enable more quick organic synthesis process development with the assistance of theoretic calculation.
of different substrates and the relative condensed local electrophilic index (ωAX − ωAH). It not only allows the prediction of the reaction rate of different substrates and the design of industrial production systems but also inspires us to enable more quick organic synthesis process development with the assistance of theoretic calculation.



 Please wait while we load your content...
Please wait while we load your content...