2,5-Furandicarboxaldehyde as a bio-based crosslinking agent replacing glutaraldehyde for covalent enzyme immobilization†
Abstract
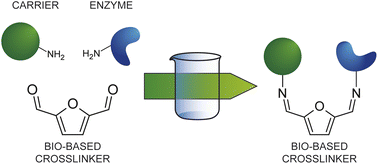
In the quest for a bio-based and safer substitute for glutaraldehyde, we have investigated 2,5 diformylfuran (DFF) as bifunctional crosslinking agent for the covalent immobilization of glucoamylase on amino-functionalized methacrylic resins. Immobilization experiments and systematic comparison with glutaraldehyde at four different concentrations for the activation step showed that DFF leads to comparable enzymatic activities at all tested concentrations. Continuous flow experiment confirms a similar long term stability of the immobilized formulations obtained with the two crosslinkers. The NMR study of DFF in aqueous solution evidenced a much simpler behaviour as compared to glutaraldehyde, since no enolic forms can form and only a mono-hydrated form was observed. Unlike in the case of glutaraldehyde, DFF reacts covalently with the primary amino groups via imine bond formation only. Nevertheless, the stability of the covalent immobilization was confirmed also at acidic pH (4.5), most probably because of the higher stability of the imine bonds formed with the aromatic aldehydes. In terms of toxicity DFF has the advantage of being poorly soluble in water and, more importantly, poorly volatile as compared to glutaraldehyde, which displays severe respiratory toxicity. We have performed preliminary ecotoxicity assays using Aliivibrio fischeri, a marine bacterium, evidencing comparable behaviour (below the toxicity threshold) for both dialdehydes at the tested concentrations.


 Please wait while we load your content...
Please wait while we load your content...