Covalently linked benzothiadiazole-fullerene adducts for organic optoelectronic devices: synthesis and characterization†
Abstract
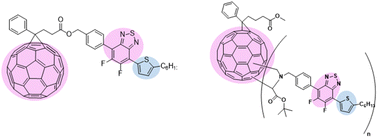
Fullerene adducts have attracted attention in a variety of applications including organic optoelectronic devices. In this regard, we have designed a covalently linked donor–acceptor dyad comprising a fluorobenzothiadiazole-thiophene (BTF2-Th) unit with the electron acceptor fullerene in an Acceptor–Donor–Acceptor (A–D–A) molecular arrangement. We synthesized and characterized two new covalently bonded benzothiadiazole-based fullerene molecules, mono-adduct, 7 (benzothiadiazole : PC61BM = 1 : 1, anchored terminally via esterification reaction) and multi-adduct, 10-I (benzothiadiazole : PC61BM = n : 1, where n ≥ 1, attached directly to the fullerene core via the Prato reaction) using different synthetic strategies. A broadening of the UV-visible spectra of the modified fullerene derivative with strong absorption from 350 to 500 nm and at low wavelengths is observed as compared to PC61BM. A suitable bandgap, good electronic conductivity, and appreciable solubility in solvents suggest their utility in optoelectronic devices. The mono-adduct 7 showed two-order higher electron mobility as compared to bis-adduct 10-I due to retention of extended conjugation in fullerene, as in the case of PC61BM. Experimentally determined optical properties and energy levels of the fullerene adducts were found to be in good agreement and supported by theoretical calculations. The presence of BTF2 affects the ground state dipole moments as well as the absorption strengths, most noticeable in the case of two attached BTF2 moieties. The HOMO and LUMO levels are found to be localized on the fullerene cage with the extension of the HOMO to the BTF2 unit more and the same is noticed in ground state dipole moment in the side-chain functionalized structure. Such structural arrangement provides easy charge transfer between acceptor and donor units to allow a concomitant effect of favorable optoelectronic properties, energy levels of the frontier orbitals, effective exciton dissociation, and charge transport which may reduce processing complexity to advance single material-based future optoelectronic devices.


 Please wait while we load your content...
Please wait while we load your content...