Decarboxylative ring-opening of 2-oxazolidinones: a facile and modular synthesis of β-chalcogen amines†
Abstract
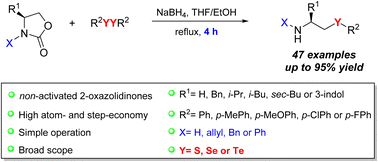
We report herein the synthesis of primary and secondary β-chalcogen amines through the regioselective ring-opening reaction of non-activated 2-oxazolidinones promoted by in situ generated chalcogenolate anions. The developed one-step protocol enabled the preparation of β-selenoamines, β-telluroamines and β-thioamines with appreciable structural diversity and in yields of up to 95%.


 Please wait while we load your content...
Please wait while we load your content...