A disulphide bond-mediated hetero-dimer of a hemoprotein and a fluorescent protein exhibiting efficient energy transfer†
Abstract
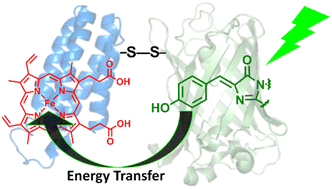
Artificial protein hetero-dimerization is one of the promising strategies to construct protein-based chemical tools. In this work, cytochrome b562, an electron transfer hemoprotein, and green fluorescent protein (GFP) mutants with cysteine residues added to their surfaces were conjugated via a pyridyl disulphide-based thiol–disulfide exchange reaction. The eight hetero-dimers, which have cysteine residues at different positions to form the disulphide bonds, were obtained and characterized by gel-electrophoresis, mass spectrometry and size exclusion chromatography. The fluorescence properties of the hetero-dimers were evaluated by fluorescence spectroscopy and fluorescence lifetime measurements. Efficient photoinduced energy transfer from the GFP chromophore to the heme cofactor was observed in each of the hetero-dimers. The energy transfer efficiency is strongly dependent on the cross-linking residues, reaching 96%. Furthermore, the estimated Förster distance and the structure-based maximum possible distances of the donor and acceptor suggest that one of the hetero-dimers has a rigid protein–protein structure with favourable properties for energy transfer. The disulphide bond-mediated protein hetero-dimerization is useful for screening functional protein systems towards further developments.


 Please wait while we load your content...
Please wait while we load your content...