Synthesis and pharmacological activities of azo dye derivatives incorporating heterocyclic scaffolds: a review
Abstract
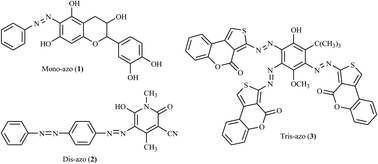
Nowadays, there is significant interest in the synthesis of heterocycle-incorporated azo dye derivatives as potential scaffolds in the pharmaceutical sector. The pharmaceutical or drug industries need a simplistic synthesis approach that can afford a wide range of azo dye derivatives. The incorporation of the heterocyclic moiety into the azo dye scaffold has improved the bioactive properties of the target derivatives. The various biological and pharmacological applications of drugs such as anti-fungal, anti-tuberculosis, anti-viral, anti-inflammatory, anti-cancer, anti-bacterial, DNA binding, and analgesic properties can be easily tuned by introducing heterocyclic moieties. To date, continuous efforts are being made in the search for more potent, new, and safe synthetic methodologies for azo dye derivatives. This review presents a brief discussion of the facile synthetic approaches and the relevance of the title compound and its derivatives towards various biological activities. Thus, the synthesis of azo dye derivatives incorporating heterocyclic scaffolds such as imidazole, pyrazole, thiazole, oxazolone, thiophene, pyrrole, benzothiazole and quinoline moieties and their pharmacological applications are discussed briefly.
- This article is part of the themed collection: 2022 Reviews in RSC Advances


 Please wait while we load your content...
Please wait while we load your content...