The role of curing temperature and reactive aluminum species on characteristics of phosphate geopolymer
Abstract
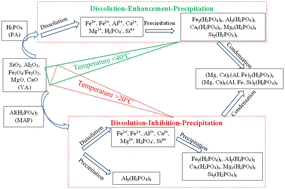
The reaction of an acid phosphate with ferro/aluminosilicate materials is a slow-setting process at room temperature that requires several days to harden. Thus, various setting accelerators are generally used to achieve quick setting and demolding in a short period. This work aims to evaluate the benefits of phosphoric acid-containing soluble aluminum and heat curing on accelerating the reaction kinetic and strength development of phosphate geopolymers. The diluted phosphoric acid (PA, 50 wt%) and acid aluminum phosphate (PA, 50 wt%, Al/P = 1/3) solutions were prepared to activate volcanic ash, and the samples were cured at 20, 40, and 60 °C to produce the phosphate geopolymer binder. The phosphate geopolymer's reaction kinetics, mechanical properties, mineralogy, and microstructure were evaluated. The results revealed that when volcanic ash was activated with diluted phosphoric acid, the reaction mechanism that prevailed was the dissolution–enhancement–precipitation–condensation, and was also fostered when the heat curing was applied. While for the acid aluminum phosphate-activated volcanic ash, the mechanism is dissolution–inhibition–precipitation–condensation. That difference in reaction mechanism led to a higher compressive strength improvement at an early age (1 d, 3 d) for room temperature cured acid aluminum phosphate activated volcanic ash. In contrast, phosphoric acid-activated volcanic ash phosphate geopolymer developed a higher compressive strength at a late age (28 d). Moreover, heat curing is the most crucial parameter having a beneficial effect on compressive strength development as compared to acid aluminum phosphate activating solution.


 Please wait while we load your content...
Please wait while we load your content...