Efficient and practical synthesis of monoalkyl oxalates under green conditions†
Abstract
Monoalkyl oxalates are among the most important building blocks being applied to the synthesis of a variety of significant classes of compounds or applied to various cutting-edge reactions. However, their commercial availability is limited. Their synthetic methods are also limited because of the difficulty to synthesize them, and those hitherto reported are carried out in organic solvents often with the use of toxic reagents with mostly low to modest yields. Here we have developed practical synthesis of monoalkyl oxalates in aqueous media by applying the highly efficient selective monohydrolysis reactions of symmetric diesters which we reported previously. The best conditions apply an aqueous NaOH solution with relatively nontoxic THF or acetonitrile as a co-solvent at around 0–5 °C. The procedures are simple and environmentally friendly without requiring toxic or expensive reagents, yet yielding the corresponding half-esters in high yields with high purities. All the half-esters prepared here are stable over a long period of time. Therefore, our studies are expected to offer practical green methods for the synthesis of monoalkyl oxalates.
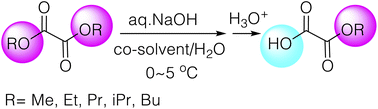
- This article is part of the themed collection: 2022 RSC Advances Popular Advances Collection


 Please wait while we load your content...
Please wait while we load your content...