Oxazoline scaffold in synthesis of benzosiloxaboroles and related ring-expanded heterocycles: diverse reactivity, structural peculiarities and antimicrobial activity†
Abstract
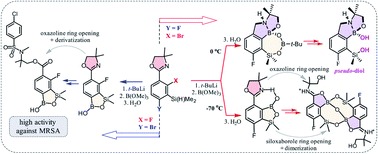
Two isomeric benzosiloxaborole derivatives 3a and 5a bearing fluorine and 4,4-dimethyl-2-oxazolin-2-yl substituents attached to the aromatic rings were obtained. Both compounds were prone to hydrolytic cleavage of the oxazoline ring after initial protonation or methylation of the nitrogen atom. The derivative 3c featuring N-methylammoniumalkyl ester functionality was successfully subjected to N-sulfonylation and N-acylation reactions to give respective derivatives which demonstrates its potential for modular synthesis of structurally extended benzosiloxaboroles. Compound 5c bearing N-ammoniumalkyl ester underwent conversion to a unique macrocyclic dimer due to siloxaborole ring opening. Furthermore, an unexpected 4-electron reduction of the oxazoline ring occurred during an attempted synthesis of 5a. The reaction gave rise to an unprecedented 7-membered heterocyclic system 4a comprising a relatively stable B–O–B–O–Si linkage and stabilized by an intramolecular N–B coordination. It could be cleaved to derivative 4c bearing BOH and SiMe2OH groups which acts as a pseudo-diol as demonstrated by formation of an adduct with Tavaborole. Apart from the multinuclear NMR spectroscopy characterization, crystal structures of the obtained products were determined in many cases by X-ray diffraction. Investigation of biological activity of the obtained compounds revealed that derivatives 3e and 3f with pendant N-methyl arylsulfonamide groups exhibit high activity against Gram-positive cocci such as methicillin-sensitive Staphylococcus aureus ATCC 6538P, methicillin-resistant S. aureus (MRSA) ATCC 43300 as well as the MRSA clinical strains, with MIC values in the range of 3.12–6.25 mg L−1. These two compounds also showed activity against Enterococcus faecalis ATCC 29212 and Enterococcus faecium ATCC 6057 (with MICs of 25–50 mg L−1). The results of the antimicrobial activity and cytotoxicity studies indicate that 3e and 3f can be considered as potential antibacterial agents, especially against S. aureus MRSA.


 Please wait while we load your content...
Please wait while we load your content...