Electrophilic halogenations of propargyl alcohols: paths to α-haloenones, β-haloenones and mixed β,β-dihaloenones†
Abstract
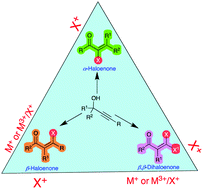
The Meyer–Schuster rearrangement of propargyl alcohols or alkynols leading to α,β-unsaturated carbonyl compounds is well known. Yet, electrophilic halogenations of the same alkynols and their alkoxy, ester and halo derivatives are inconspicuous. This review on the halogenation reactions of propargyl alcohols and derivatives intends to give a perspective from its humble direct halogenation beginning to the present involving metal catalysis. The halogenation products of propargyl alcohols include α-fluoroenones, α-chloroenones, α-bromoenones and α-iodoenones, as well as β-haloenones and symmetrical and mixed β,β-dihaloenones. They are, in essence, tri and tetrasubstituted alkenes carrying halo-functionalization at the α- or β-carbon. This is a potential stepping stone for further construction towards challenging substituted alkenones via Pd-catalysed coupling reactions.


 Please wait while we load your content...
Please wait while we load your content...