Precise immunological evaluation rationalizes the design of a self-adjuvanting vaccine composed of glycan antigen, TLR1/2 ligand, and T-helper cell epitope†
Abstract
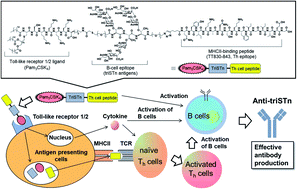
Sialyl-Tn (STn), overexpressed on various tumors, has been investigated for its application in anti-cancer vaccine therapy. However, Theratope, an STn-based vaccine, failed in the phase III clinical trial due to poor immunogenicity and epitope suppression by the foreign carrier protein. We therefore developed a self-adjuvanting STn based-vaccine, a conjugate of clustered STn (triSTn) antigen, TLR1/2 ligand (Pam3CSK4), and T-helper (Th) cell epitope, and found that this three-component self-adjuvanting vaccine effectively resulted in the production of anti-triSTn IgG antibodies. We herein analyzed immune responses induced by this self-adjuvanting vaccine in detail. We newly synthesized two-component vaccines, i.e., Pam3CSK4- or Th epitope-conjugated triSTn, as references to evaluate the immune-stimulating functions of Pam3CSK4 and Th epitope. Immunological evaluation of the synthesized vaccine candidates revealed that Pam3CSK4 was essential for antibody production, indicating that the uptake of triSTn antigen by antigen-presenting cells (APCs) was promoted by the recognition of Pam3CSK4 by TLR1/2. The function of the Th epitope was also confirmed. Th cell activation was important for boosting antibody production and IgG subclass switching. Furthermore, flow cytometric analyses of immune cells, including T cells, B cells, dendritic cells, and other monocytes, were first employed in the evaluation of self-adjuvanting vaccines and revealed that the three-component vaccine was able to induce antigen-specific immune responses for efficient antibody production without excessive inflammatory responses. Importantly, the co-administration of Freund's adjuvants was suggested to cause excessive myeloid cell accumulation and decreased plasma cell differentiation. These results demonstrate that vaccines can be designed to achieve the desired immune responses via the bottom-up construction of each immune element.


 Please wait while we load your content...
Please wait while we load your content...