Aggregation induced emission based fluorenes as dual-channel fluorescent probes for rapid detection of cyanide: applications of smartphones and logic gates†
Abstract
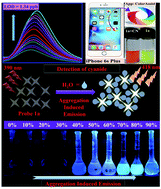
Rational modification of molecular structure by incorporating electron donating groups can play a potential role for designing aggregation induced emission (AIE) active fluorescent probes. Based on this principle, fluorescent probes (1a–c) were synthesized, and they displayed excellent aggregation induced emission (AIE) behavior in a H2O/DMF (4 : 1, v/v) mixture due to restrictions in intramolecular charge transfer (ICT). As a comparison, probe 1d was synthesized by installing an electron withdrawing (–NO2) group that surprisingly quenched the aggregation behaviour. Additionally, AIE active probes 1a–c displayed a highly sensitive dual channel (fluorometric and colorimetric) response towards rapid detection of CN−, which is an active toxic material. Probes 1a–c showed selectively enhanced fluorescence emission behavior towards CN− with detection limits of 1.34 ppb, 1.38 ppb, and 1.54 ppb, respectively. The sensing mechanism involves Michael type adduct formation due to the nucleophilic addition reaction of cyanide with probes and was confirmed through 1H NMR titration experiments. In contrast, probe 1d containing an electron withdrawing moiety showed insensitivity towards CN−. Therefore, this study provides the efficient strategy to induce AIE character in fluorescent probes and expands the mechanistic approach toward the sensing of toxic CN−.


 Please wait while we load your content...
Please wait while we load your content...