Oxa-376 and Oxa-530 variants of β-lactamase: computational study uncovers potential therapeutic targets of Acinetobacter baumannii†
Abstract
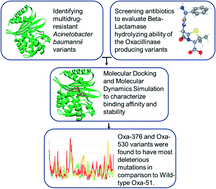
Antimicrobial resistance is a major global health crisis, resulting in thousands of deaths each year. Antibiotics' effectiveness against microorganisms deteriorates over time as multidrug resistance (MDR) develops, which is exacerbated by irregular antibiotic use, poor disease management, and the evasive nature of bacteria. The World Health Organization has recognized multidrug resistance as a critical public health concern, and Acinetobacter baumannii has been at the center of attention due to its ability to develop multidrug resistance (MDR). It generally produces carbapenem-hydrolyzing oxacillinase, which has been identified as the primary source of beta-lactam resistance in MDR bacteria. Recently, point mutations in A. baumannii have been identified as a key factor of multidrug resistance, making them a prime concern for researchers. The goal of the current work was to establish a unique way of finding multidrug-resistant variants and identify the most damaging mutations in the existing databases. We characterized the deleterious variants of oxacillinases using several computational tools. Following a thorough analysis, Oxa-376 and Oxa-530 were found to be more damaging when compared with the wild-type Oxa-51. The mutants' 3D structures were then prepared and refined with RaptorX, GalaxyRefine, and SAVES servers. Our research incorporates seven antimicrobial agents to illustrate the resistance capability of the variants of oxacillinase by evaluating binding affinity in Autodock-vina and Schrodinger software. RMSD, RMSF, Radius of gyration analysis, the solvent-accessible surface area (SASA), hydrogen bonding analysis and MM-GBSA from Molecular Dynamics Simulation revealed the dynamic nature and stability of wild-type and Oxa-376 and Oxa-530 variants. Our findings will benefit researchers looking for the deleterious mutations of Acinetobacter baumannii and new therapeutics to combat those variants. However, further studies are necessary to evaluate the mechanism of hydrolyzing activity and antibiotic resistance of these variants.


 Please wait while we load your content...
Please wait while we load your content...