Mechanofluorochromism of (D–π–)2A-type azine-based fluorescent dyes†
Abstract
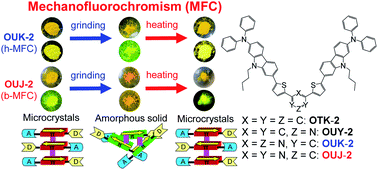
Bathochromic or hypsochromic shift-type mechanofluorochromism (b-MFC or h-MFC) was found for (D–π–)2A-type azine-based fluorescent dyes OUY-2, OUK-2, and OUJ-2 possessing intramolecular charge-transfer (ICT) characteristics from two (diphenylamino)carbazole–thiophene units as D (electron-donating group)–π (π-conjugated bridge) moieties to a pyridine, pyrazine, or triazine ring as A (electron-withdrawing group): grinding of the recrystallized dyes induced red or blue shifts of the fluorescent colors, that is, bathochromic or hypsochromic shifts of the fluorescence maximum wavelengths (λfl-solidmax). The degrees of MFC evaluated by the absolute value of differences (Δλfl-solidmax) in λfl-solidmax before and after grinding of the recrystallized dyes increased in the order of OUY-2 (+7 nm) < OUK-2 (−17 nm) < OUJ-2 (+45 nm), so that OUJ-2 exhibits obvious b-MFC, but OUK-2 exhibits h-MFC. X-ray powder diffraction (XRD) and differential scanning calorimetry (DSC) demonstrated that the recrystallized dyes were in the crystalline state but the ground dyes were in the amorphous state. When the ground solids were heated above their crystallization temperatures (Tc), the colors and fluorescent colors recovered to the original ones before grinding or converted to other ones, that is, heating the ground solids in the amorphous state induced the recrystallization to recover the original microcrystals or to form other microcrystals due to polymorph transformation. However, (D–π–)2Ph-type fluorescent dye OTK-2 having a phenyl group as a substitute for the azine rings exhibited non-obvious MFC. Molecular orbital (MO) calculations indicated that the values of the dipole moments (μg) in the ground state were 4.0 debye, 1.4 debye, 3.2 debye, and 2.9 debye for OTK-2, OUY-2, OUK-2, and OUJ-2, respectively. Consequently, on the basis of experimental results and MO calculations, we have demonstrated that the MFC of the (D–π–)2A-type azine-based fluorescent dyes is attributed to reversible switching between the crystalline state of the recrystallized dyes and the amorphous state of the ground dyes with changes in the intermolecular dipole–dipole and π–π interactions before and after grinding. Moreover, this work reveals that (D–π–)2A fluorescent dyes possessing dipole moments of ca. 3 debye as well as moderate or intense ICT characteristics make it possible to activate the MFC.


 Please wait while we load your content...
Please wait while we load your content...