Synthesis of potent and selective HDAC6 inhibitors led to unexpected opening of a quinazoline ring†
Abstract
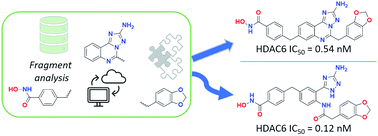
Histone deacetylase (HDAC) inhibitors are highly involved in the regulation of many pharmacological responses, which results in anti-inflammatory and anti-cancer effects. In the present work, chemoinformatic analyses were performed to obtain two potent and selective aminotriazoloquinazoline-based HDAC6 inhibitors. We unexpectedly obtained an aminotriazole from a water-driven ring opening of the triazoloquinazoline scaffold. Both compounds were evaluated as HDAC6 inhibitors, resulting in subnanomolar inhibitory activity and high selectivity with respect to class I HDAC1 and HDAC8. Importantly, the compounds were about 3- and 15-fold more potent compared to the reference compound trichostatin A. Additionally, the predicted binding modes were investigated with docking. Considering that the aminotriazole scaffold has never been embedded into the chemical structure of HDAC6 inhibitors, the present study suggests that both the aminotriazoloquinazoline and aminotriazole classes of compounds could be excellent starting points for further optimization of potential anticancer compounds, introducing such novel groups into a relevant and new area of investigation.


 Please wait while we load your content...
Please wait while we load your content...