Deracemization of 1-phenylethanols in a one-pot process combining Mn-driven oxidation with enzymatic reduction utilizing a compartmentalization technique†
Abstract
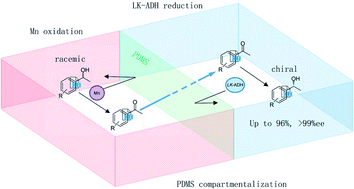
Racemic 1-phenylethanols were converted into enantiopure (R)-1-phenylethanols via a chemoenzymatic process in which manganese oxide driven oxidation was coupled with enzymatic biotransformation by compartmentalization of the reactions, although the two reactions conducted under mixed conditions are not compatible due to enzyme deactivation by Mn ions. Achiral 1-phenylethanol is oxidized to produce acetophenone in the interior chamber of a polydimethylsiloxane thimble. The acetophenone passes through the membrane into the exterior chamber where enantioselective biotransformation takes place to produce (R)-1-phenylethanol with an enantioselectivity of >99% ee and with 96% yield. The developed sequential reaction could be applied to the deracemization of a wide range of methyl- and chloro-substituted 1-phenylethanols (up to 93%, >99% ee). In addition, this method was applied to the selective hydroxylation of ethylbenzene to afford chiral 1-phenylethanol.


 Please wait while we load your content...
Please wait while we load your content...