Electrospray ionization of native membrane proteins proceeds via a charge equilibration step†
Abstract
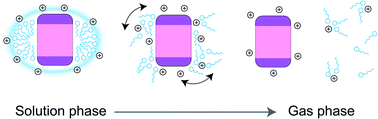
Electrospray ionization mass spectrometry is increasingly applied to study the structures and interactions of membrane protein complexes. However, the charging mechanism is complicated by the presence of detergent micelles during ionization. Here, we show that the final charge of membrane proteins can be predicted by their molecular weight when released from the non-charge reducing saccharide detergents. Our data indicate that PEG detergents lower the charge depending on the number of detergent molecules in the surrounding micelle, whereas fos-choline detergents may additionally participate in ion–ion reactions after desolvation. The supercharging reagent sulfolane, on the other hand, has no discernible effect on the charge of detergent-free membrane proteins. Taking our observations into the context of protein-detergent interactions in the gas phase, we propose a charge equilibration model for the generation of native-like membrane protein ions. During ionization of the protein-detergent complex, the ESI charges are distributed between detergent and protein according to proton affinity of the detergent, number of detergent molecules, and surface area of the protein. Charge equilibration influenced by detergents determines the final charge state of membrane proteins. This process likely contributes to maintaining a native-like fold after detergent release and can be harnessed to stabilize particularly labile membrane protein complexes in the gas phase.


 Please wait while we load your content...
Please wait while we load your content...