Li4SiO4 Doped-Li7P2S8I solid electrolytes with high lithium stability synthesised using liquid-phase shaking†
Abstract
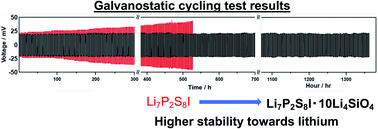
In this study, mechanical milling and liquid-phase shaking are used to synthesise 3Li2S·P2S5 LiI·xLi4SiO4 (Li7P2S8I·xLi4SiO4) solid electrolytes. When mechanical milling is used, the electrolyte samples doped with 10 mol% of Li4SiO4 (Li7P2S8I·10Li4SiO4) have the highest ionic conductivity at ∼25–130 °C. When liquid-phase shaking is used, they exhibit a relatively high conductivity of 0.85 mS cm−1 at ∼20 °C, and low activation energy for conduction of 17 kJ mol−1. A cyclic voltammogram shows that there are no redox peaks between −0.3 and +10 V, other than the main peaks near 0 V (v.s. Li/Li+), indicating a wide electrochemical window. The galvanostatic cycling test results demonstrate that the Li7P2S8I·10Li4SiO4 has excellent long-term cycling stability in excess of 680 cycles (1370 h), indicating that it is highly compatible with Li. Thus, Li7P2S8I solid electrolytes doped with Li4SiO4 are synthesised using the liquid-phase shaking method for the first time and achieve a high ionic conductivity of 0.85 mS cm−1 at 25 °C. This work demonstrates the effects of Li4SiO4 doping, which can be used to improve the ionic conductivity and stability against Li anodes with Li7P2S8I solid electrolytes.


 Please wait while we load your content...
Please wait while we load your content...