Diol responsive viscosity increase in a cetyltrimethylammonium bromide/sodium salicylate/3-fluorophenylboronic acid micelle system
Abstract
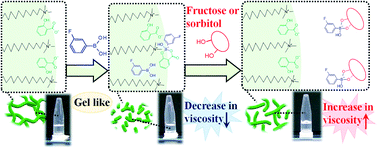
We report a novel smart micellar system utilising a phenylboronic acid (PBA) derivative whose viscosity increases on adding diol compounds such as sugar or sugar alcohol. We prepared a typical worm-like micelle (WLM) system in 100 mM cetyltrimethylammonium bromide (CTAB)/70 mM sodium salicylate (NaSal), which showed high zero-shear viscosity (η0). Upon the addition of 20 mM 3-fluorophenylboronic acid (3FPBA) to the WLM system, η0 decreased by 1/300 that of the system without 3FPBA. Furthermore, upon the addition of 1.12 M fructose (Fru) and 1.12 M sorbitol (Sor) to the CTAB/NaSal/3FPBA system, η0 increased by 50-fold and 30-fold, respectively. 19F NMR spectral results of the systems using 4-fluorosalicylic acid (FSal) instead of NaSal demonstrated that the FSal/3FPBA-complex interacts with CTAB. Moreover, the addition of sugar or sugar alcohol to the micellar system leads to a decrease in the amount of FSal/3FPBA-complex interacting with CTA+ and an increase in the amount of 3FPBA/Fru or Sor-complex, which does not interact with CTA+. These changes in molecular interactions induce the elongation of the WLMs and increase the viscosity of the system. This system utilises the competitive cyclic ester bond between the NaSal/3FPBA and 3FPBA/sugar or sugar alcohol to induce viscosity changes.


 Please wait while we load your content...
Please wait while we load your content...