Evaluation of mixed transition metal (Co, Mn, and Cu) oxide electrocatalysts anchored on different carbon supports for robust oxygen reduction reaction in neutral media†
Abstract
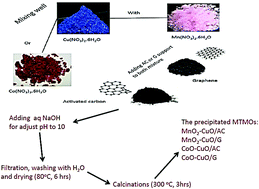
Oxygen reduction reaction (ORR) remains a pivotal factor in assessing the overall efficiency of energy conversion and storage technologies. A promising family of ORR electrocatalysts is mixed transition-metal oxides (MTMOs), which have recently gained a growing research interest. In this study, we developed MTMOs with different compositions (designated as AxB3−xO4; A = Cu, B = Co or Mn) anchored on two different carbon supports (activated carbon Vulcan XC-72 (AC) and graphene (G)) for catalyzing ORR in neutral media. Four different MTMO electrocatalysts (i.e., MnO2–CuO/AC, CoO–CuO/AC, CoO–CuO/G, and MnO2–CuO/G) were synthesized by a simple and scalable co-precipitation method. We documented the morphology and electrocatalytic properties of MTMO electrocatalysts using transmission and scanning electron microscopy, X-ray diffraction (XRD), X-ray photoelectron spectrometer (XPS), energy dispersive X-ray (EDX), and electrochemical techniques. Generally, MTMOs exhibited remarkably high ORR electrocatalytic activity with MTMOs anchored on an activated carbon support outperforming their respective MTMOs anchored on a graphene support, highlighting the importance of the catalyst support in determining the overall ORR activity of electrocatalysts. MnO2–CuO/AC has the highest diffusion limiting current density (j) value of 4.2 mA cm−2 at −600 mV (vs. SHE), which is ∼1.1–1.7-fold higher than other tested electrocatalysts (i.e., 3.9, 3.5, and 2.7 mA cm−2 for CoO–CuO/AC, CoO–CuO/G, and MnO2–CuO/G, respectively), and slightly lower than Pt/C (5.1 mA cm−2) at the same potential value. Moreover, all electrocatalysts exhibited good linearity and parallelism of the Koutechy–Levich (K–L) plots, suggesting that ORR followed first-order reaction kinetics with the number of electrons involved being close to four. Benefiting from their remarkable ORR electrochemical activities and low cost, our results reveal that non-precious MTMOs are efficient enough to replace expensive Pt for broad applications in energy conversion and electrocatalysis in neutral media, such as microbial fuel cells.


 Please wait while we load your content...
Please wait while we load your content...