Modulating catalytic activity of a modified flavin analogue via judicially positioned metal ion toward aerobic sulphoxidation†
Abstract
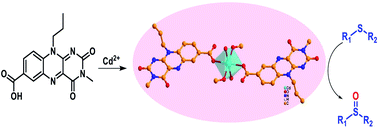
This manuscript describes the synthesis, spectroscopic and crystallographic characterization of a cadmium complex of 10-propoylisoalloxazine-7-carboxylic acid (Flc-Cd). Catalytic activity of Flc-Cd towards aerobic sulphoxidation reaction was investigated in the presence of L-ascorbic acid as the reducing agent. Notably the neutral un-metalated flavin analogue did not show any significant catalytic activity. The design strategy for Flc provides a close proximity of the metal centre to the flavin core without compromising the catalytic site thereby assisting the product formation when compared to unmetallated Flc. Minor enantioselectivity is also observed in cases where unsymmetrical sulphides were used; indicative of the possible involvement of chiral L-ascorbic acid in the intermediate formation.
- This article is part of the themed collection: Emerging Investigators Series


 Please wait while we load your content...
Please wait while we load your content...