Unification of Ullmann and Kharasch coupling: acid promoted CuI catalysed C–N coupling protocols under ligand, base and solvent free conditions†
Abstract
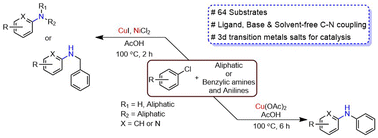
The copper catalysed C–N cross coupling reaction (Ullmann coupling) is one of the most extensively explored reactions in organic synthesis. Despite its notable advantages of being less toxic, and the use of inexpensive and abundant Cu metal, a continuous methodological development over the last century has been reported for a significant improvement of the general applicability of the reaction to a remarkable extent. Herein, we have demonstrated a significantly upgraded Ullmann reaction for C–N cross couplings of aryl/alkyl chlorides. The method works in completely opposite conditions and requires acid media instead of basic media. Moreover, the presented method is significantly influenced by the presence of various 3d transition metals, which work as a promoter and drastically reduce the reaction time. Furthermore, the reaction conditions significantly increase the catalytic activity of CuI, as well as the functional group tolerance of the reaction. Aliphatic amines (1° and 2°), benzylic amine, and anilines are well supported with arylchlorides. In conclusion, the presented method efficiently couples a wide variety of aryl/alkyl chlorides that are economical but difficult to activate, and the reaction works in the complete absence of ligand, base and solvent media.


 Please wait while we load your content...
Please wait while we load your content...