Halogen bond promoted aryl migration of allylic alcohols under visible light irradiation†
Abstract
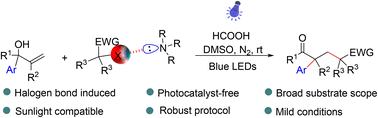
Considerable efforts have recently been devoted to exploring the migration reactions of allyl alcohols by employing transition-metal catalysts or photocatalysts. Herein, a simple and catalyst-free radical addition/1,2-aryl migration cascade process of allyl alcohol was developed under visible light irradiation. A mechanism investigation reveals that halogen bonds play an essential role in initiating the cascade process. Not only the well-known perfluoro iodide but also other electron-deficient organohalides were proven to be effective halogen bond donors to participate in this reaction. Furthermore, the protocol allows for late-stage functionalization of bioactive molecules, is compatible with sunlight irradiation and can be scaled up to gram quantities of products. The simplicity, practicality and broad substrate scope characteristics of this method highlight the power of photocatalysis through a halogen-bonded complex and hopefully will inspire further development of green and low-cost photocatalytic systems based on noncovalent interactions.


 Please wait while we load your content...
Please wait while we load your content...