endo-5-Norbornene-2,3-dimethanol-promoted asymmetric Heck/Suzuki cascade reaction of N-(2-bromophenyl)acrylamides†
Abstract
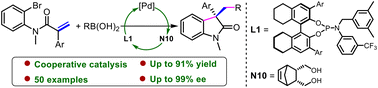
A highly enantioselective palladium-catalyzed Heck/Suzuki cascade reaction of N-(2-bromophenyl)acrylamides has been developed. The key to the success of this transformation was found to be the endo-5-norbornene-2,3-dimethanol helping prevent transmetalation of the aryl-palladium complex. The reaction was shown to tolerate a wide range of functional groups and provide straightforward access to an array of oxindoles with excellent ee values.


 Please wait while we load your content...
Please wait while we load your content...