Radical cyclization/bis(pentafluoroethylation) of 1,6-dienes using HCF2CF3-derived CuCF2CF3†
Abstract
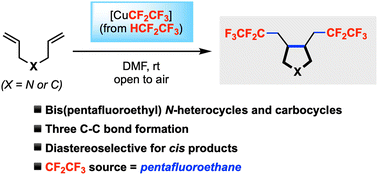
We herein describe a domino radical cyclization/bis(pentafluoroethylation) of 1,6-dienes for the synthesis of new classes of pyrrolidine and cyclopentane derivatives containing two C2F5 groups. This reaction is efficient for constructing three carbon–carbon bonds in one step. The reagent [CuCF2CF3] is prepared from inexpensive pentafluoroethane and the reactions conveniently run at room temperature in open air. Moderate to good diastereoselectivies can be obtained favouring the cis products with X-ray structural proof.


 Please wait while we load your content...
Please wait while we load your content...