Triton B-promoted regioselective intramolecular addition of enolates to tethered ynamides for the construction of 8-membered O-heterocycles†
Abstract
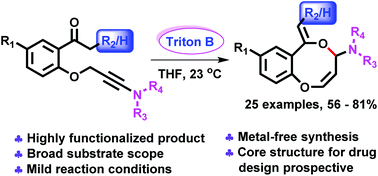
The synthesis of natural products containing eight-membered heterocycles has proved to be highly challenging. In this contribution, an environmentally benign synthesis of benzo-1,5 dioxocines via ynamides is reported. A new class of ynamides was prepared by using a green and mild synthetic pathway. The eight-membered heterocycles fused to aryl groups were obtained from propargylic ynamides by treatment with Triton B via addition of enolates to N-allenamide ethers. Deprotonation of the propargylic methylene group occurred before enolization of the methyl ketone because of the polarization of the triple bond by the amide moiety. Regioselective proximal O-alkylation on the N-allenamide provided shelf-stable eight-membered-heterocycles substituted by an amide in an economic fashion with no byproducts. This transformation is applicable to a wide range of ynamides, expanding significantly the chemical space of benzo-1,5 dioxocines crucial from a drug design perspective.


 Please wait while we load your content...
Please wait while we load your content...